At Extremity Care we offer a diverse portfolio of cell and tissue products - including placental allografts, xenografts, acellular dermis, and conservative wound care products.
We focus on technologies that support the repair, reconstruction, replacement, or supplementation of the patient’s own tissue. Through our dedicated lab and AATB accredited tissue bank we are at the forefront of cell and tissue engineering. We strive to be good stewards of the precious gift of tissue donation.
With our careLOG™ shipping logistics process, we provide just-in-time handling of cell and tissue products. careLOG™ contains the tissue, product information and the billing-related documents needed to request reimbursement.
Abstract, Articles and Posters
-

Abstract
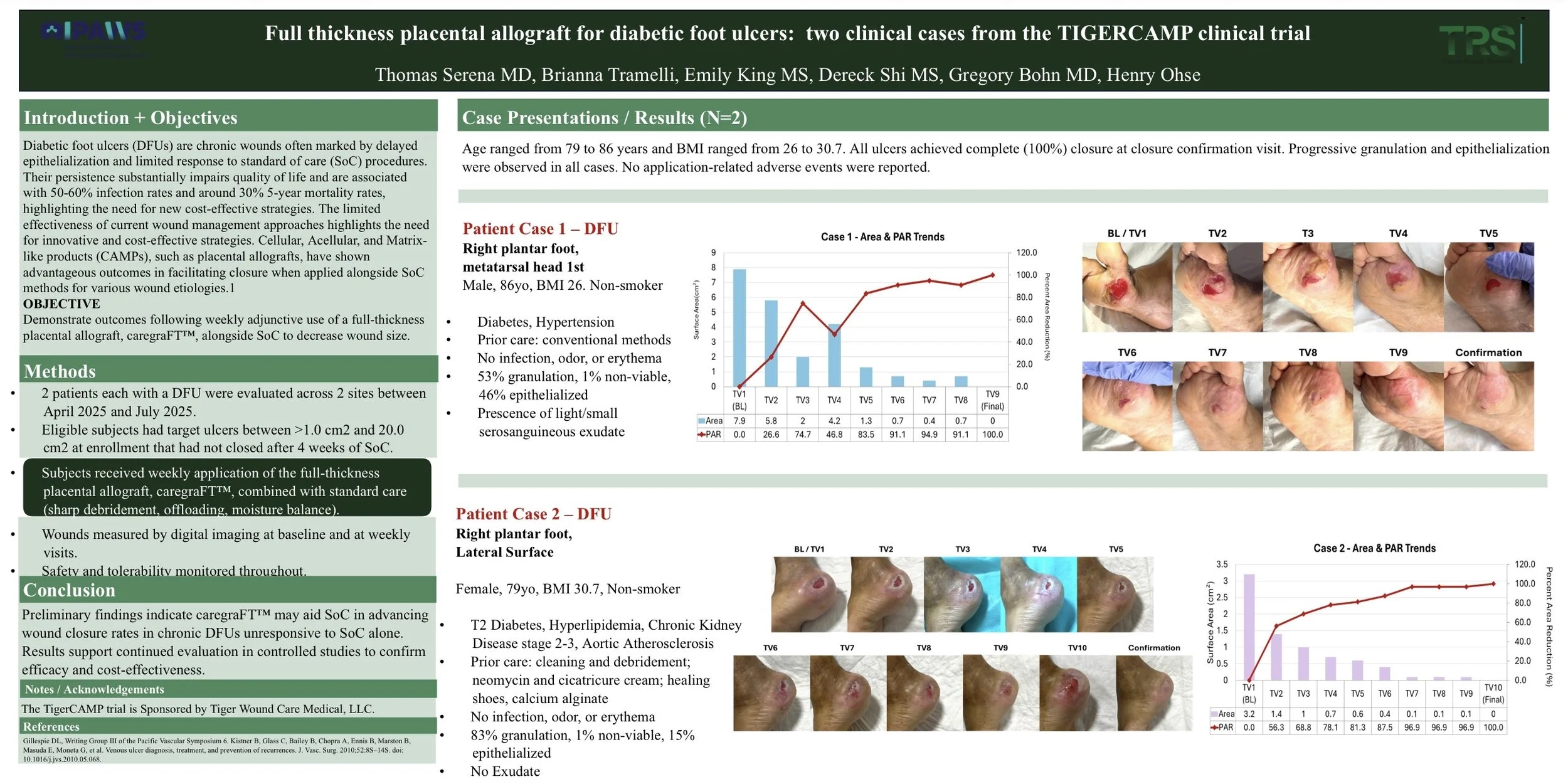
Background: Diabetic foot ulcers (DFUs) are chronic wounds often marked by delayed epithelialization and limited response to standard of care (SOC) procedures. Their persistence substantially impairs quality of life and are associated with 50-60% infection rates and around 30% 5-year mortality rates, highlighting the need for new cost-effective strategies.
-

Abstract
A multicenter, prospective randomized controlled modified dual platform trial evaluating several cellular, acellular, and matrix-like products (CAMPs) and standard of care versus standard of care alone in the management of nonhealing diabetic foot ulcers and venous leg ulcers (TIGERCAMP)
-

Abstract
This randomized controlled modified platform trial is designed to assess the efficacy of cellular, acellular, and matrix-like products alongside SOC in the closure of pressure ulcers. The trial will initially review two CAMPs: amnion chorion amnion placental allograft (ACA; ACApatch™, Tiger Wound Care Medical, LLC, Conshohocken, PA, USA) and full-thickness placental mem-brane allograft (FT; caregraFT™, Tiger Wound Care Medical, LLC, Conshohocken, PA, USA). However, the modified platform design allows for additional products to be included with protocol amendment.
-

Abstract
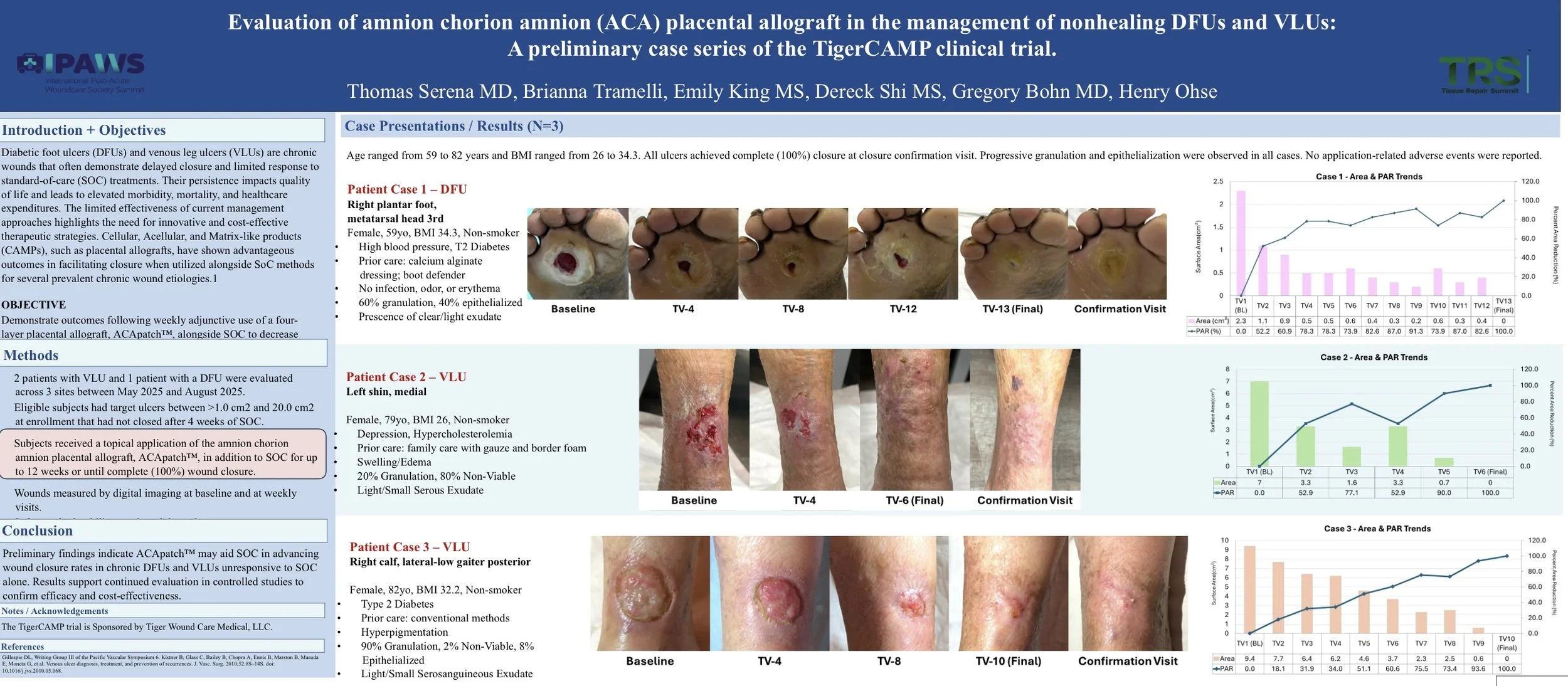
Background: Diabetic foot ulcers (DFUs) and venous leg ulcers (VLUs) are chronic wounds that often demonstrate delayed closure and limited response to standard-of-care (SOC) procedures. Their persistence impacts quality of life and leads to elevated morbidity, mortality, and healthcare expenditures. The limited effectiveness of current management approaches highlights the need for additional, innovative, and cost-effective strategies.
-

Abstract
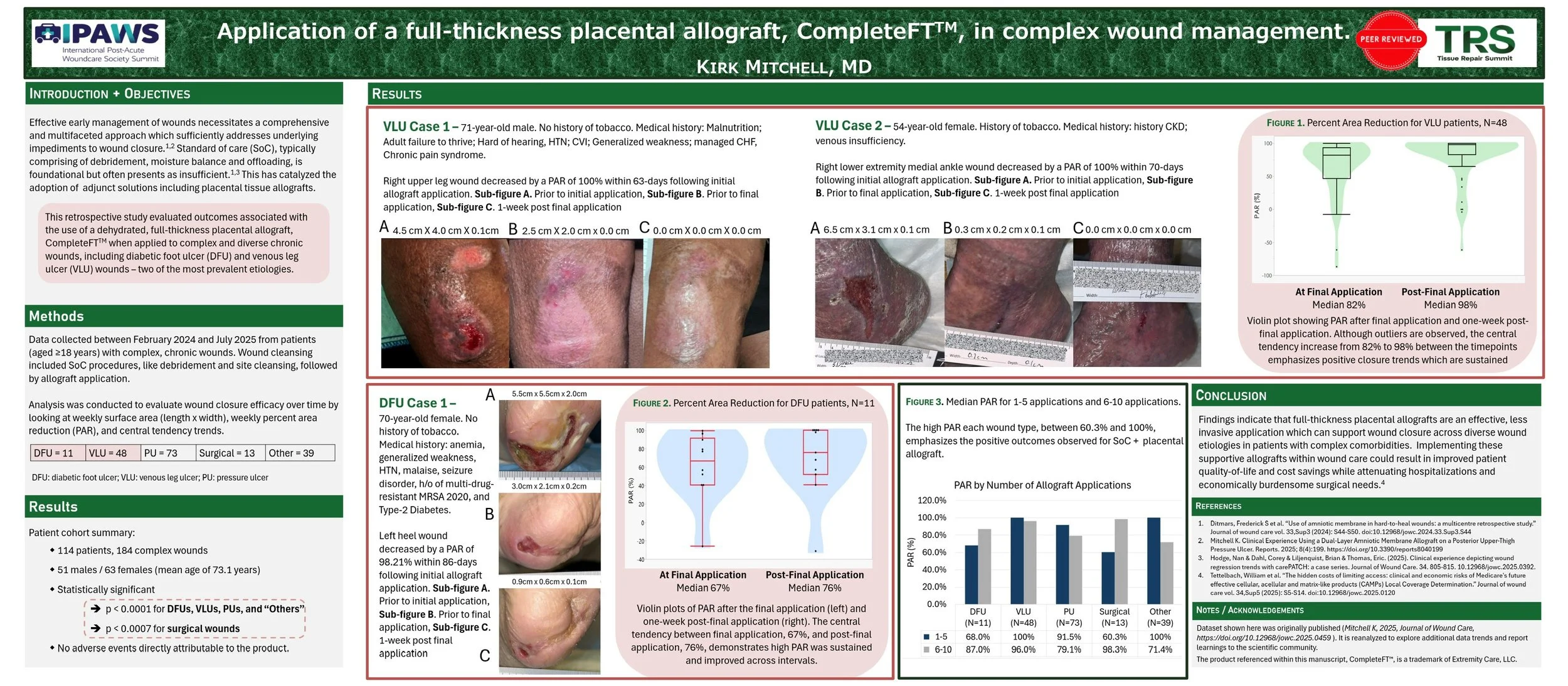
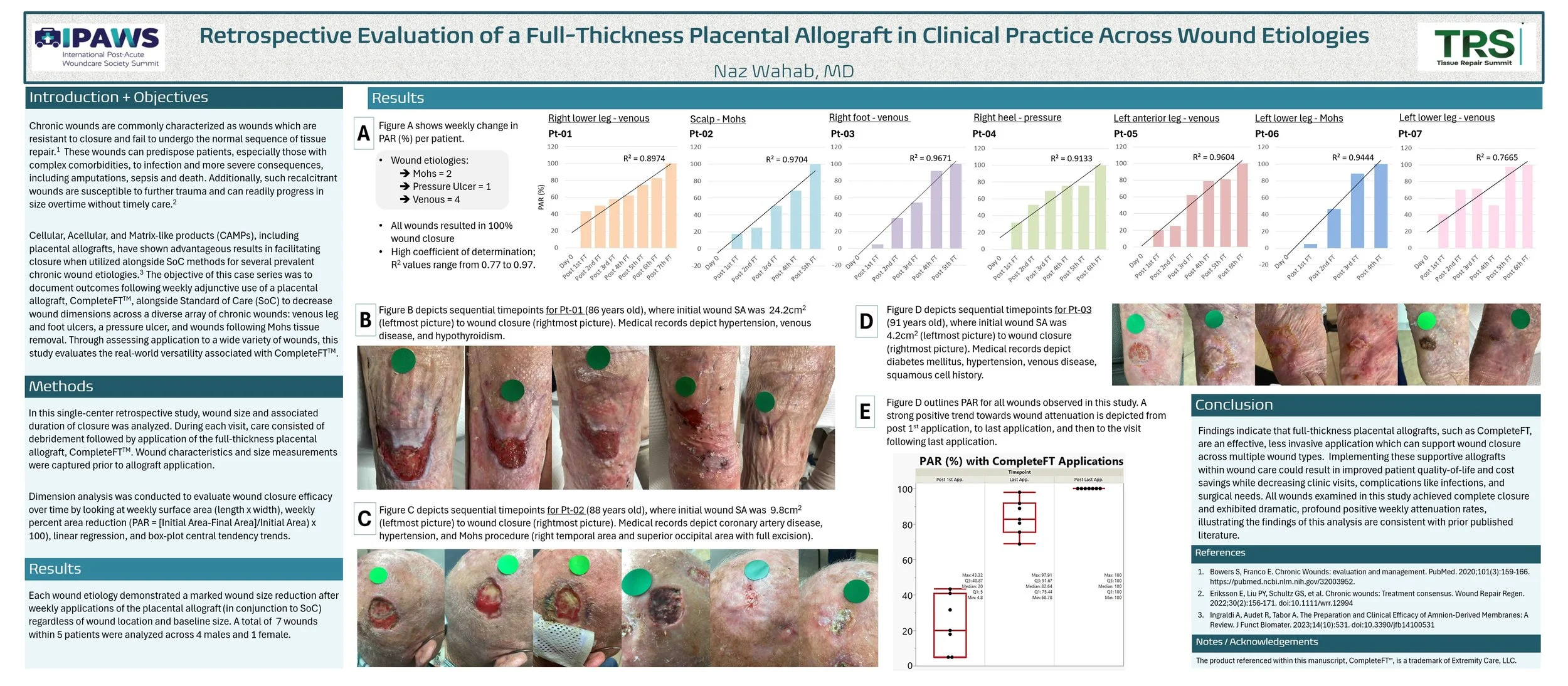
Aim: The complex nature of chronic wounds requires care that is not limited to general Standard of Care (SoC) approaches like debridement, wound dressings, negative pressure therapy, hyperbaric oxygen therapy, and of the like. Cellular, acellular, and matrix-like products (CAMPs), such as amniotic membranes, are a prospective solution to these challenges. A growing body of efficacy and safety evidence suggests placental derived allografts have successfully been applied alongside SoC in heterogenous populations with complex comorbidities. The present analysis examines wound closure performance across wounds of varying pathologies characterized by prior lack of improvement under SoC alone.
Podium Presenation