Expanding Access.
Empowering Outcomes.
OneBioTech is committed to making regenerative medicine a standard of care—not a specialty. By equipping clinicians with biologic solutions and expert support, we help them expand treatment options for patients with complex, chronic, or non-healing wounds.
Abstract, Articles and Posters
-

Abstract
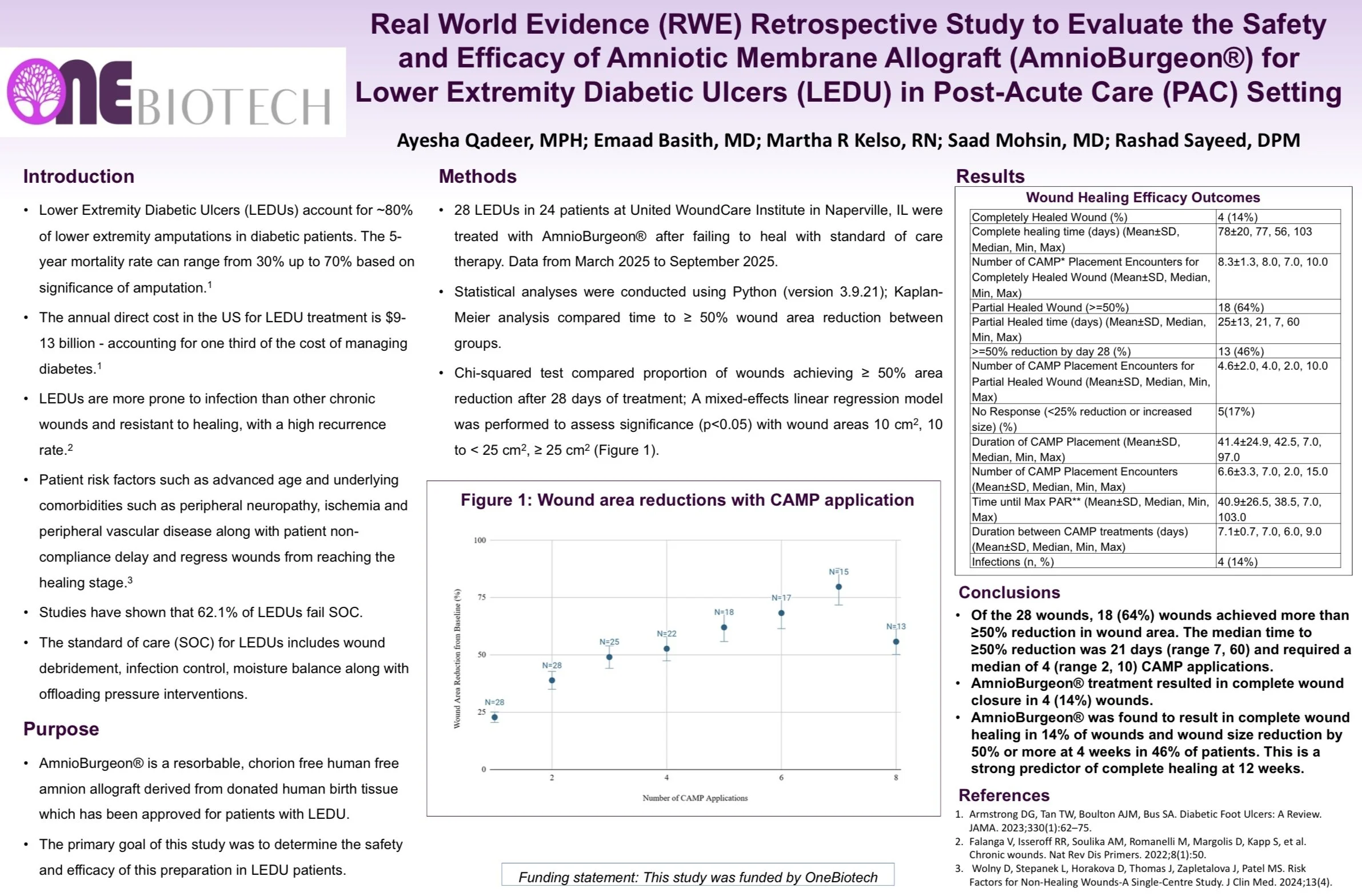
Real world evidence retrospective study to evaluate the safety and efficacy of an amniotic membrane allograft for lower extremity diabetic ulcers in a post-acute care setting.
-

Abstract
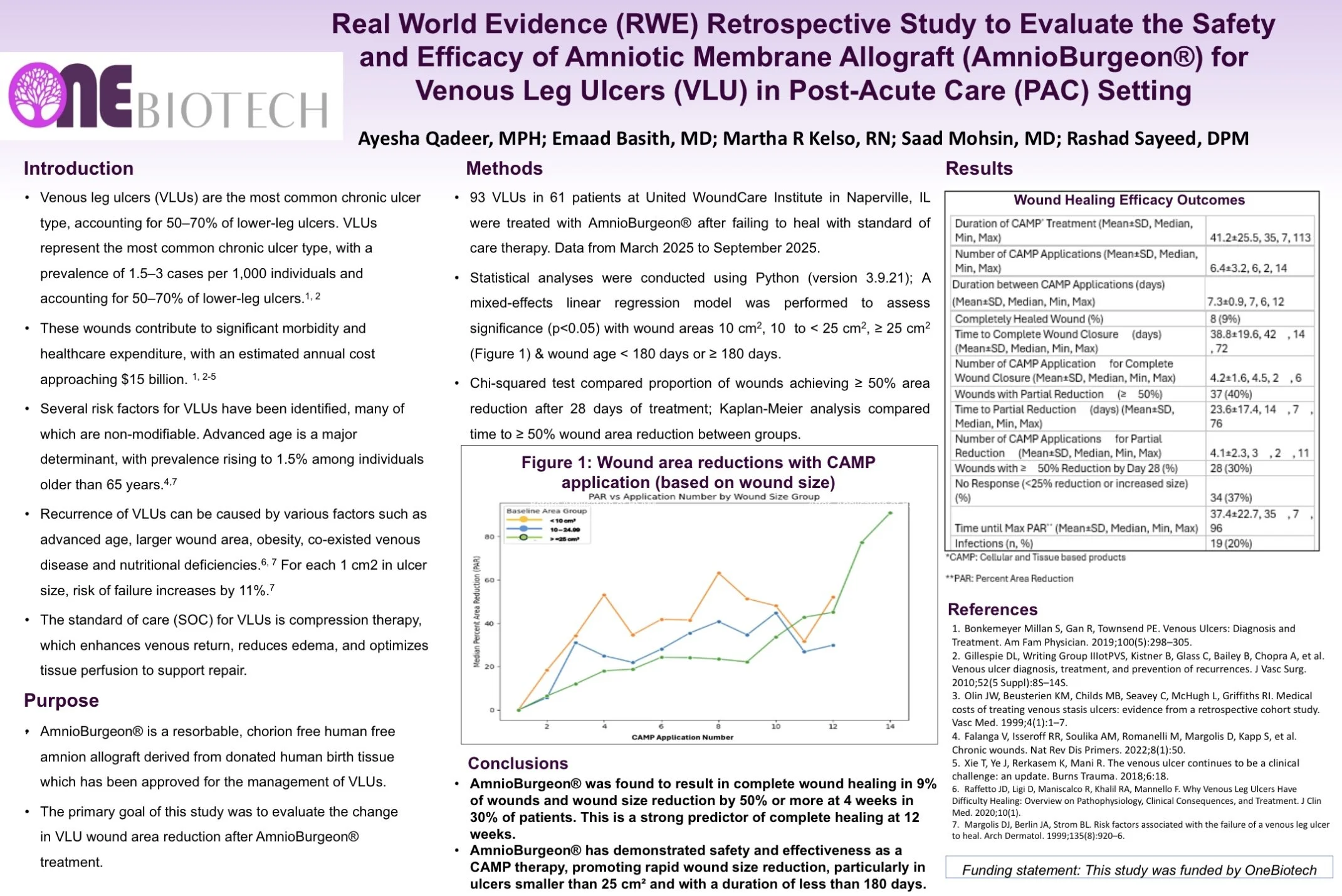
Real world evidence retrospective study to evaluate the safety and efficacy of an amniotic membrane allograft for venous leg ulcers in a post-acute care setting
-

Article
Aims: To evaluate the reduction of wound size in venous leg ulcers (VLUs) after amniotic membrane allograft (AmnioBurgeon (OneBiotech, USA)) treatment.
Methods: Single-center retrospective database study of patients treated with an amniotic membrane allograft for VLUs. Wound area was measured prior to each allograft application, and the change in wound area after each application was evaluated over the progression of therapy.
-

Article
The physiological process of wound healing in ulcers that have stalled is a complex, multi-step process for restoring injured tissue.1
When this process is interrupted, it can lead to either over-healing or the formation of a chronic wound.2
By definition, a chronic wound is one that has not healed after receiving four weeks of standard care.3
Podium Presentation